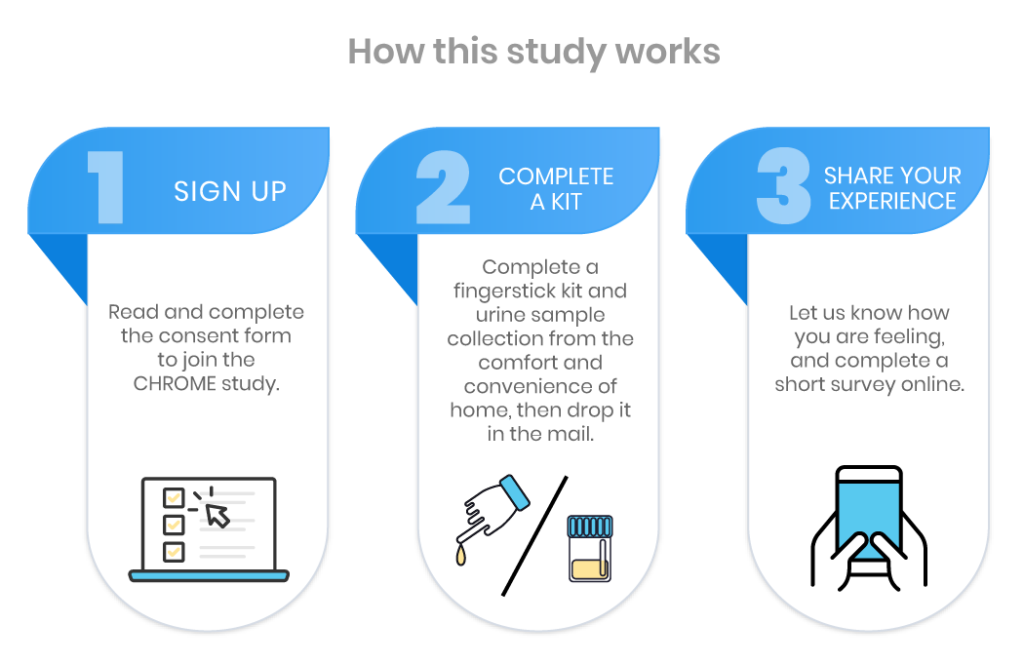
Study participants will complete a kit + questionnaire to establish their CFS activity. A small fraction of participants will be asked to complete a second kit + questionnaire 1-2 weeks following the first sample collection. Participants may give permission to review their medical records, serving as another data source, but will not be required to do so. Providing medical record access helps researchers make connections between each participant’s blood and urine samples and their medical history.
Researchers will use self-reported information and medical records to understand the collected samples better by making connections between each participant’s blood and urine chemistry and their medical history.